8:16 AM Reaction types | ||||||||||||
 Reaction typesOxidation and reduction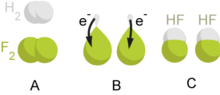 Redox reactions can be understood in terms of transfer of electrons from one involved species (reducing agent) to another (oxidizing agent). In this process, the former species is oxidized and the latter is reduced, thus the term redox.
Though An example of a redox reaction is:
Here I2 is reduced to I– and S2O32– (thiosulfate anion) is oxidized to S4O62–. Which of the involved reactants would be reducing or oxidizing agent can be predicted from the electronegativity of their elements. Elements with low electronegativity, such as most metals,
easily donate electrons and oxidize – they are reducing agents. On the
contrary, many ions with high oxidation numbers, such as H2O2, MnO− The number of electrons donated or accepted in a redox reaction can be predicted from electron configuration of the reactant element. Elements are trying to reach the low-energy noble gas configuration, and therefore alkali metals and halogens will donate and accept one electron, respectively, and the noble gases themselves are chemically inactive. An important class of redox reactions are the electrochemical reactions, where the electrons from the power supply are used as a reducing agent. These reactions are particularly important for the production of chemical elements, such as chlorine or aluminium. The reverse process in which electrons are released in redox reactions and can be used as electrical energy is possible and is used in the batteries.
ComplexationIn complexation reactions, several ligands react with a metal atom to form a coordination complex. This is achieved by providing lone pairs of the ligand into empty orbitals of the metal atom and forming dipolar bonds. The ligands are Lewis bases, they can be both ions and neutral molecules, such as carbon monoxide, ammonia or water. The number of ligands that react with a central metal atom can be found using the 18-electron rule, saying that the valence shells of a transition metal will collectively accommodate 18 electrons, whereas the symmetry of the resulting complex can be predicted with the crystal field theory and ligand field theory. Complexation reactions also include ligand exchange, in which one or more ligands are replaced by another, and redox processes which change the oxidation state of the central metal atom.Acid-base reactionsAcid-base reactions involve transfer of protons from one molecule (acid) to another (base). Here, acids act as proton donors and bases as acceptors.
The associated proton transfer results in the so-called conjugate acid and conjugate base. The reverse reaction is possible, and thus the acid/base and conjugated base/acid are always in equilibrium. The equilibrium is determined by the acid and base dissociation constants (Ka and Kb) of the involved substances. A special case of the acid-base reaction is the neutralization where an acid and a base, taken at exactly same amounts, form a neutral salt. Acid-base reactions can have different definitions depending on the acid-base concept employed. Some of the most common are:
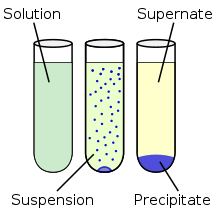 Precipitation PrecipitationPrecipitation is the formation of a solid in a solution or inside another solid during a chemical reaction. It usually takes place when the concentration of dissolved ions exceeds the solubility limit and forms an insoluble salt. This process can be assisted by adding a precipitating agent or by removal of the solvent. Rapid precipitation results in an amorphous or microcrystalline residue and slow process can yield single crystals. The latter can also be obtained by recrystallization from microcrystalline salts.
|
 |
 |
|
|
SN1 mechanism
|
SN2 mechanism
|

steps of an
SN2 reaction.
The
nucleophile
is green and
the leaving
group is red
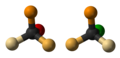
causes
stereo
inversion
(Walden
inversion)
The SN1 reaction proceeds in two steps. First, the leaving group is eliminated creating a carbocation. This is followed by a rapid reaction with the nucleophile.
In the SN2 mechanism, the nucleophile forms a transition state with the attacked molecule, and only then the leaving group is cleaved. These two mechanisms differ in the stereochemistry of the products. SN1 leads to the non-stereospecific addition and does not result in a chiral center, but rather in a set of geometric isomers (cis/trans). In contrast, a reversal (Walden inversion) of the previously existing stereochemistry is observed in the SN2 mechanism.
Electrophilic substitution is the counterpart of the nucleophilic substitution in that the attacking atom or molecule, an electrophile, has low electron density and thus a positive charge. Typical electrophiles are the carbon atom of carbonyl groups, carbocations or sulfur or nitronium cations. This reaction takes place almost exclusively in aromatic hydrocarbons, where it is called electrophilic aromatic substitution. The electrophile attack results in the so-called σ-complex, a transition state in which the aromatic system is abolished. Then, the leaving group, usually a proton, is split off and the aromaticity is restored. An alternative to aromatic substitution is electrophilic aliphatic substitution. It is similar to the nucleophilic aliphatic substitution and also has two major types, SE1 and SE2

In the third type of substitution reaction, radical substitution, the attacking particle is a radical. This process usually takes the form of a chain reaction, for example in the reaction of alkanes with halogens. In the first step, light or heat disintegrates the halogen-containing molecules producing the radicals. Then the reaction proceeds as an avalanche until two radicals meet and recombine.

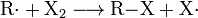
- Reactions during the chain reaction of radical substitution
Addition and elimination
The addition and its counterpart, the elimination, are reactions which change the number of substituents on the carbon atom, and form or cleave multiple bonds. Doubletriple bonds can be produced by eliminating a suitable leaving group. Similar to the nucleophilic substitution, there are several possible reaction mechanisms which are named after the respective reaction order. In the E1 mechanism, the leaving group is ejected first, forming a carbocation. The next step, formation of the double bond, takes place with elimination of a proton (deprotonation). The leaving order is reversed in the E1cb mechanism, that is the proton is split off first. This mechanism requires participation of a base. Because of the similar conditions, both reactions in the E1 or E1cb elimination always compete with the SN1 substitution. and
 |
 |
|
|
E1 elimination
|
E1cb elimination
|

The E2 mechanism also requires a base, but there the attack of the base and the elimination of the leaving group proceed simultaneously and produce no ionic intermediate. In contrast to the E1 eliminations, different stereochemical configurations are possible for the reaction product in the E2 mechanism, because the attack of the base preferentially occurs in the anti-position with respect to the leaving group. Because of the similar conditions and reagents, the E2 elimination is always in competition with the SN2-substitution.
The counterpart of elimination is the addition where double or triple bonds are converted into single bonds. Similar to the substitution reactions, there are several types of additions distinguished by the type of the attacking particle. For example, in the electrophilic addition of hydrogen bromide, an electrophile (proton) attacks the double bond forming a carbocation, which then reacts with the nucleophile (bromine). The carbocation can be formed on either side of the double bond depending on the groups attached to its ends, and the preferred configuration can be predicted with the Markovnikov's rule. This rule states that "In the heterolytic addition of a polar molecule to an alkene or alkyne, the more electronegative (nucleophilic) atom (or part) of the polar molecule becomes attached to the carbon atom bearing the smaller number of hydrogen atoms."
If the addition of a functional group takes place at the less substituted carbon atom of the double bond, then the electrophilic substitution with acids is not possible. In this case, one has to use the hydroboration–oxidation reaction, where in the first step, the boron atom acts as electrophile and adds to the less substituted carbon atom. At the second step, the nucleophilic hydroperoxide or halogen anion attacks the boron atom.
While the addition to the electron-rich alkenes and alkynes is mainly electrophilic, the nucleophilic addition plays an important role for the carbon-heteroatom multiple bonds, and especially its most important representative, the carbonyl group. This process is often associated with an elimination, so that after the reaction the carbonyl group is present again. It is therefore called addition-elimination reaction and may occur in carboxylic acid derivatives such as chlorides, esters or anhydrides. This reaction is often catalyzed by acids or bases, where the acids increase by the electrophilicity of the carbonyl group by binding to the oxygen atom, whereas the bases enhance the nucleophilicity of the attacking nucleophile.
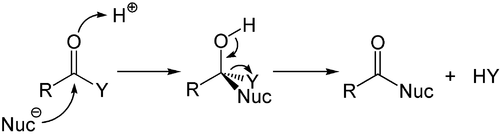
Nucleophilic addition of a carbanion or another nucleophile to the double bond of an alpha, beta unsaturated carbonyl compound can proceed via the Michael reaction, which belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C-C bonds.
Some additions which can not be executed with nucleophiles and electrophiles, can be succeeded with free radicals. As with the free-radical substitution, the radical additionfree-radical polymerization proceeds as a chain reaction, and such reactions are the basis of the
Other organic reaction mechanisms

Diels-Alder reaction

Diels-Alder reaction
In a rearrangement reaction, the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. These include hydride shift reactions such as the Wagner-Meerwein rearrangement, where a hydrogen, alkyl or aryl group migrates from one carbon to a neighboring carbon. Most rearrangements are associated with the breaking and formation of new carbon-carbon bonds. Other examples are sigmatropic reaction such as the Cope rearrangement.
Cyclic rearrangements include cycloadditions and, more generally, pericyclic reactions, wherein two or more double bond-containing molecules form a cyclic molecule. An important example of cycloaddition reaction is the Diels–Alder reactiondiene and a substituted alkene to form a substituted cyclohexene system (the so-called [4+2] cycloaddition) between a conjugated
Whether or not a certain cycloaddition would proceed depends on the electronic orbitals of the participating species, as only orbitals with the same sign of wave function will overlap and interact constructively to form new bonds. Cycloaddition is usually assisted by light or heat. These perturbations result in different arrangement of electrons in the excited state of the involved molecules and therefore in different effects. For example, the [4+2] Diels-Alder reactions can be assisted by heat whereas the [2+2] cycloaddition is selectively induced by light. Because of the orbital character, the potential for developing stereoisomeric products upon cycloaddition is limited, as described by the Woodward-Hoffmann rules.
Biochemical reactions
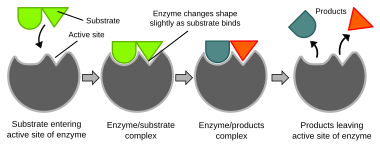
Biochemical reactions are mainly controlled by enzymes. These proteins can specifically catalyze a single reaction, so that reactions can be controlled very precisely. The reaction takes place in the active site, a small part of the enzyme which is usually found in a cleft or pocket lined by amino acid residues, and the rest of the enzyme is used mainly for stabilization. The catalytic action of enzymes relies on several mechanisms including the molecular shape ("induced fit"), bond strain, proximity and orientation of molecules relatively to the enzyme, proton donation or withdrawal (acid/base catalysis), electrostatic interactions and many others.
The biochemical reactions that occur in living organisms are collectively known as metabolism. Among the most important its mechanisms is the anabolism, in which different DNA and enzyme-controlled processes result in the production of large molecules such as proteins and carbohydrates from smaller units. Bioenergeticsglucose, which can be produced by plants via photosynthesis or assimilated from food. All organisms use this energy to produce adenosine triphosphate (ATP), which can then be used to energise other reactions studies the sources of energy for such reactions. An important energy sources is
| Total comments: 1 | ||||||
| ||||||
 sufficient for many purposes, these descriptions are not
precisely correct. Oxidation is better defined as an increase in oxidation number,
and reduction as a decrease in oxidation number. In practice, the
transfer of electrons will always change the oxidation number, but
there are many reactions that are classed as "redox" even though no
electron transfer occurs (such as those involving
sufficient for many purposes, these descriptions are not
precisely correct. Oxidation is better defined as an increase in oxidation number,
and reduction as a decrease in oxidation number. In practice, the
transfer of electrons will always change the oxidation number, but
there are many reactions that are classed as "redox" even though no
electron transfer occurs (such as those involving